
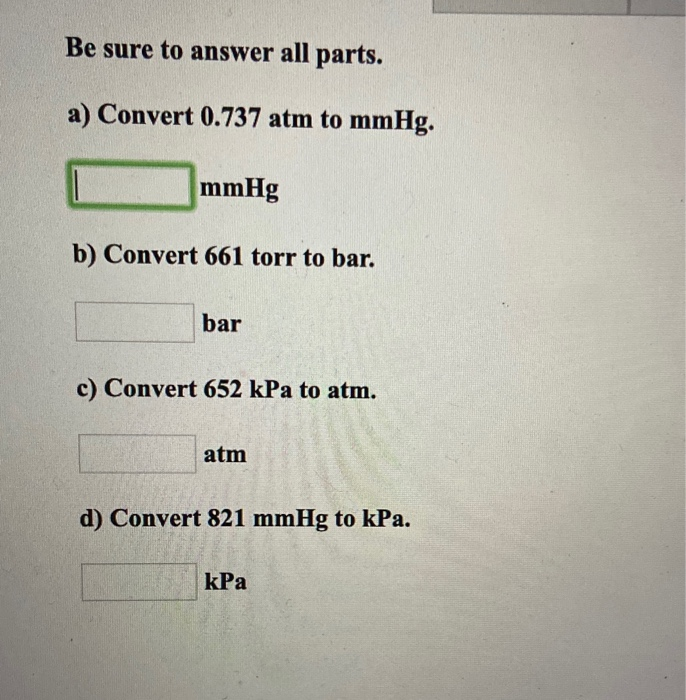
(b) A mixture of He and O 2 gases is used by deep sea divers. (a) What is the partial pressure (in atm) of each gas in the atmosphere? Problem #19: Our atmosphere is a mixture of gases (roughly 79% N 2, 20% O 2 and 1%Ar).

Since the right-hand side is constant, the answer is B. Problem #18: Which of the following is constant for 1 mole of any ideal gas? Convert 17.15 g and 17.85 g to their respective moles and divide moles of CO 2 by moles of Kr. Is the above gram ratio also a 1.83 to 1 molar ratio? Please be aware that "gram fractions" is not a standard term.Ĭomment: Based on this ratio (0.458/0.250) the CO 2:Kr molar ratio is 1.83 to 1. How many grams of CO 2 and how many grams of Kr were initially present?ġ) Calculate the mole fractions of CO 2 and Kr: After the CO 2 is completely removed by absorption with NaOH(s) the pressure in the container is 0.250 atm. Since Kr is expensive, you wish to recover it from the mixture. Problem #17: A mixture of CO 2 and Kr weighs 35.0 g and exerts a pressure of 0.708 atm in its container. Since moles is a direct measure of the number of molecules, we do not have to determine how many molecules this is.Ģ) Determine volume of SO 2 that holds 0.089.

X = 0.089276229 mol (I kept some guard digits.) Problem #16: What volume of SO 2 at 25.0 ☌ and 1.50 atm contains the same number of molecules as 2.00 L of chlorine gas measured at STP? They are dependent only on the temperature. All vapor pressures are independent of the actual volume above the liquid. Notice that I did not reduce the vapor pressure value by one-third. Then, one-third of the gas leaked out of the container. Problem #15: In an experiment 350.00 mL of hydrogen gas was collected over water at 25.0 ☌ and 720.00 mmHg. This is the amount of moles of gas added, not the total moles. V 1 represents the volume of the flask, which does not change.Ģ) Since V 1 = V 1 and R = R, divide (a) by (b):
How many moles of gas are now in the flask?ġ) Use PV = nRT with the first set of data to get the volume of the container: The new pressure is 795.0 mm Hg and the temperature is now 26.0 ☌. The flask is now opened and more gas is added to the flask. Problem #14: A sample of gas (1.90 mol) is in a flask at 21.0 ☌ and 697.0 mm Hg. What is the percent composition of the mixture by (a) mass and by (b) volume.ġ) Calculate total moles of gases present:Ĭomment: assume that the volume of the gas mixture is 1.00 L Problem #13: A gas mixture composed of helium and argon has a density of 0.704 g/L at a 737 mmHg and 298 K. V = 24.45388 L (I kept some guard digits)Ĭomment: Titan's atmosphere is five times more dense than Earth's atmosphere. Determine its volume under the conditions of Earth's atmosphere: Assuming ideal behavior, calculate the density of Earth's atmosphere under these conditions.ġ) Let us assume the presence of one mole of gas. Earth's surface temperature is 298 K and its pressre is 1.00 atm. Problem #12: The mean molar mass of the atmosphere at the surface of the Earth is 29.0 g/mol. Determine its volume under the conditions of Titan's atmosphere: Assuming ideal behavior, calculate the density of Titan's atmosphere under these conditions.ġ) Let us assume the presence of one mole of gas.
Titan's surface temperature is 95 K and its pressure is 1.6 atm. Problem #11: The mean molar mass of the atmosphere at the surface of Titan, Saturn's largest moon is 28.6 g/mol. ChemTeam: Assorted Gas Law Problems 11-25


 0 kommentar(er)
0 kommentar(er)
